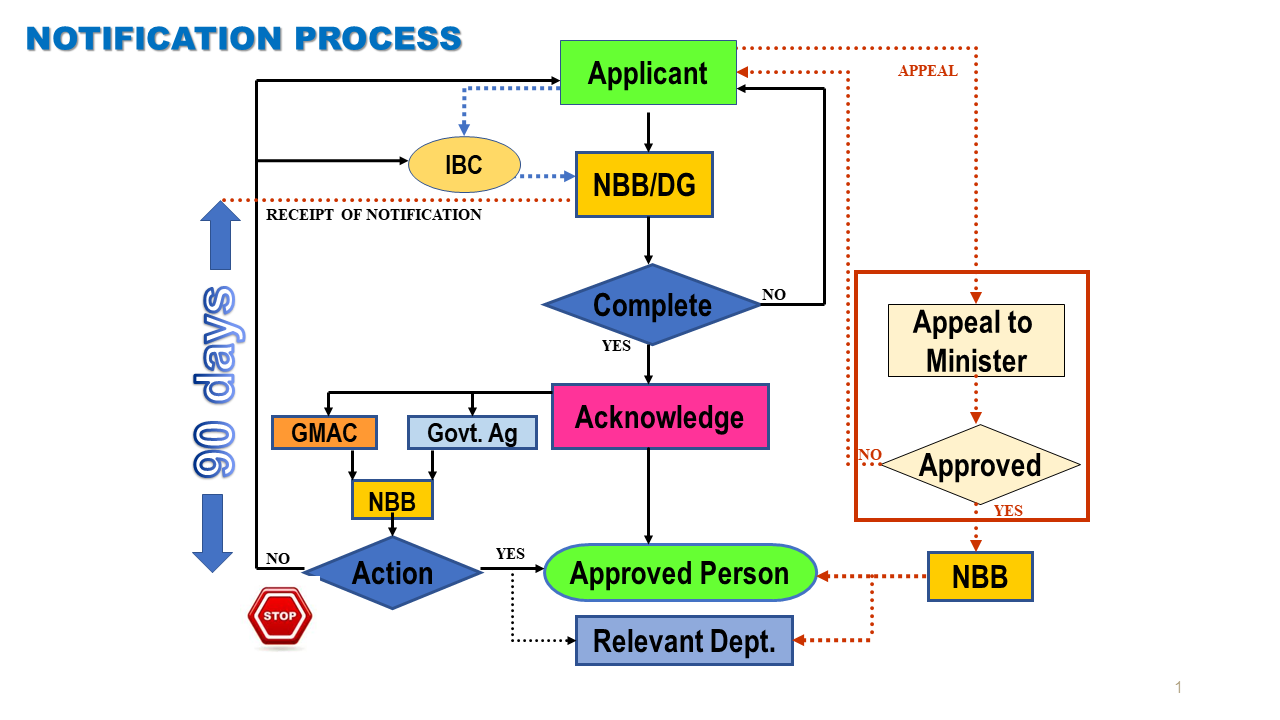
Notifications must be submitted for all contained use activities involving living modified organisms (LMOs). Contained use activities are conducted within a facility structure, installation or other physical structure that prevents LMO contact and impact to the external environment. Types of contained use activities includes research and development, production operations, manufacturing and storage.
LMOs used in contained use activities may be imported or produced locally.
If the contained use activity is for research and development purposes, the applicant's IBC must evaluate and approve of the activity beforehand by submitting the Annex 2 form (IBC Assessment of Project Proposal Involving Modern Biotechnology Activities) together with the Form E to the Department of Biosafety.
Contained use activities may begin after obtaining an acknowledgement letter from the Director General of Biosafety. However, the National Biosafety Board may impose such terms and conditions as deemed necessary after considering such notification.
Notification Process
 Fi
Fi
No fee payment
Relevant forms and documents
a) Form E (Notification for Contained Use and Import for Contained Use Activities Involving LMO for Biosafety Levels 1, 2, 3 and 4)
Description:
This form is used for controlled use activities involving LMOs
· LMOs used in contained use activities may be imported or produced locally.
· All classes of biosafety levels (Biosafety Levels 1, 2, 3 and 4) are included in this form
· If the contained use activity is for research and development purposes, the applicant's IBC must evaluate and approve of the activity beforehand by submitting the Annex 2 form (IBC Assessment of Project Proposal Involving Modern Biotechnology Activities) together with Form E.
b) Example Form E (will be upload soon) completely filled in
Description:
This is an example of Form E that has been completed and can be used as a guide to fill out the form for applicants.
c) Annex 2 (IBC Assessment of Project Proposal Involving Modern Biotechnology Activities)
Description:
This form must be submitted for contained use activities for research and development purposes. The IBC must conduct an assessment of the contained use activity by submitting the Annex 2 form together with Form E when notifying Department of Biosafety.
d) Guidelines for LMO Contained Use Activities
Description:
These guidelines provide details on the level of biosafety for contained use, how to handle different types of LMO in a safe manner, treatment of hazardous biological wastes, disposal of LMO and waste materials, transfer and transport of LMO and related materials and storage of LMO and materials related. These guidelines can be applied to all modern biotechnology research and development activities conducted in Government and non-Government laboratories, or by individuals involved in such activities. It also provides the minimum requirements for establishing facility structures to carry out LMO contained use activities, as well as identifying equipment requirements for different levels of Biosafety.





